Delivering the next stage in affordable
T cell cancer treatment through the utilization of iPS cells
Shinobi Therapeutics Co.Ltd.

Overcoming roadblocks with a revolutionary cancer treatment method
Recent years have seen a surge of interest in cell therapy, a treatment for cancer in which T cells—a type of immune cell—are harvested from patients, injected with a gene that attacks cancer, and then returned to the patient's body. In 2019, CAR-T cell therapy, a cutting-edge immunotherapy treatment for leukemia and other blood cancers, was first approved in Japan. Cell therapy’s ability to specifically target cancer cells gives it the advantage of significantly reducing damage to healthy patient tissue compared to anticancer drugs and radiation therapy.
Existing approaches to cell therapy are not without their drawbacks, however, which have prevented widespread adoption of this treatment method thus far. For one, utilizing the patient's own cells (known as autologous cells) increases treatment costs, and in many cases, the patient's T cells may already be too weak to be suitable for use.
The use of cells from another individual (known as allogeneic cells) can eliminate these disadvantages, but unfortunately creates an entirely different challenge: the patient's immune system will reject cells that are not its own, making it difficult to develop effective treatments derived from donor cells.
Creating an iPS cell-derived, rejection-evading T cell generation platform
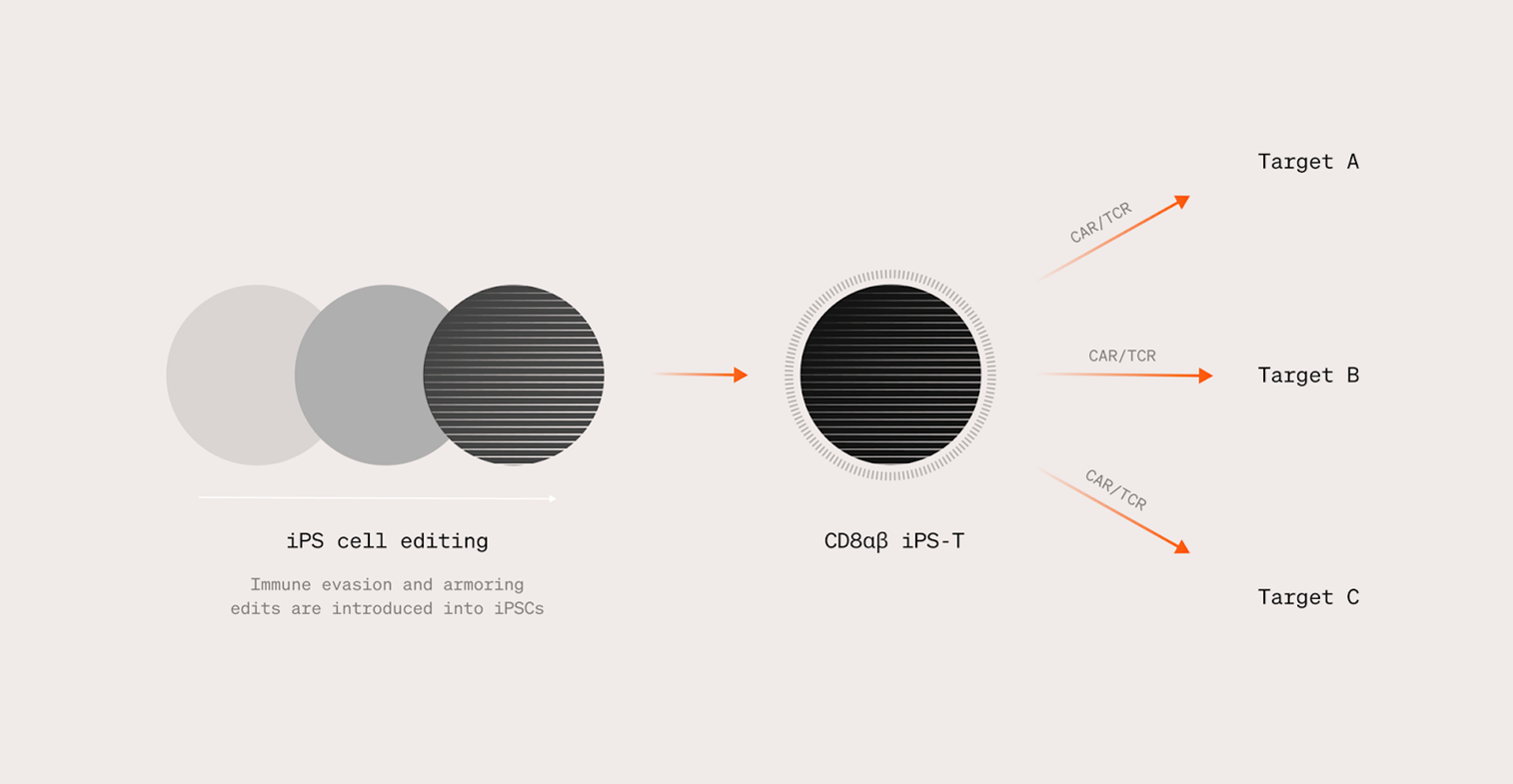
The technology being developed by Shinobi Therapeutics serves as a solution to the issues introduced thus far. By combining iPS-derived T cell therapies developed by Professor Shin Kaneko of the Center for iPS Cell Research and Application (CiRA) at Kyoto University with leading immune evasion technology invented by Professor Tobias Deuse of the University of California, San Francisco, Shinobi Therapeutics aims to create a platform for the generation of iPS cell-derived allogeneic T cells that can evade rejection by the patient's immune system. Development of the production technology for this approach is being finalized, and the company plans to begin conducting IND enabling studies in 2024.
Large-scale, low-cost production of T cells
At present, CAR-T therapy using autologous cells costs more than 30 million yen (approximately 200,000 USD) per treatment before insurance, but Shinobi Therapeutics expects that therapy utilizing the company’s immune system-evasive allogeneic T cells could slash these costs to as little as several hundred thousand to a million yen.
In addition, Shinobi Therapeutic’s platform allows for the production and storage of a large quantity of iPS cell-derived allogeneic T cells, which results in significantly shortened treatment lead times.
Towards utilization for autoimmune diseases and solid tumors
T cells produced by Shinobi Therapeutic’s platform are equipped with immune evasion capabilities, which allows them to remain effective in the patient's body over the long term. At present, cell therapies such as CAR-T are well-established only for use with blood cancers, where cancer cells are easily accessible. With their new technology, however, Shinobi Therapeutics aims to deliver therapy that is effective for solid tumors in tissues that are difficult for T cells to reach. The company is also working on technology for the instant generation of a wide variety of T cells from these iPS cells, with plans to develop this technology into a treatment for use with autoimmune diseases.
Technical and business excellence with a global presence
Shinobi Therapeutics initially began as Thyas, a Kyoto University venture founded by Shin Kaneko in 2015. In early 2022, the company raised 2.13 billion yen in Series B financing, and went on to reorganize as a subsidiary under the US-based Thyas Inc. in January 2023. In July of the same year, Thyas changed its name to Shinobi Therapeutics, where Dr. Daniel Kemp is now CEO of the US company.
In order to promote collaboration with CiRA and National Cancer Center Japan, research efforts for Shinobi Therapeutic’s iPS cell development are carried out in Kyoto, while the company’s San Francisco site handles introducing immune evasion technology to developed iPS cells, submitting clinical trial applications, and fundraising. The early relocation of Shinobi Therapeutic’s headquarters to the U.S. has been successful, with the company recently completing a $51 million (approximately 7.4 billion yen) Series A financing round in December 2023. The Kyoto and San Francisco offices are now cooperating to refine technological development initiatives while establishing a framework to compete globally from within the U.S., which stands at the forefront of gene-cell therapy treatment.

With a combination of advanced technologies born oceans apart in Kyoto and San Francisco, Shinobi Therapeutics is now striving to compete—while still only at the startup stage—in the United States, the market capital of the medical industry. Armed with enormous technological potential, the company now finds itself steadily on its way to becoming a global leader in the field of cell therapy.

President and Representative Director of
Shinobi Therapeutics Co. Ltd.: Yasumichi Hitoshi
Yasumichi graduated and obtained a medical license from Kumamoto University before earning a PhD in Immunology at the university’s graduate school. After working as an assistant at the Kumamoto University School of Medicine and the University of Tokyo Institute of Medical Science, he engaged in functional genomics research using retroviruses at Stanford University. Yasumichi then joined Rigel Pharmaceuticals, Inc. where he was involved in drug discovery research and development (identification of drug targets and preclinical studies) and as a principal investigator for new drug applications in the fields of oncology and metabolism.
Yasumichi boasts nearly 20 years of experience in drug discovery R&D within the biotech industry. Since 2017, he has been spearheading the development of immunotherapy using T cells with heightened killer activity, induced through differentiation from low immunogenicity genome-edited iPS cells at Shinobi Therapeutics.

Representative Director, COO/CFO of
Shinobi Therapeutics Co. Ltd.: Ryosuke Gonotsubo
As the head of the investment and business development support team of a general trading company, Ryosuke has promoted numerous domestic and international business investments, M&As, and venture investments in a wide range of fields. He provides comprehensive support for new business development at trading companies, including business scheme development, business plan formulation, and investment contract negotiations.
Ryosuke has experience in launching his own in-house venture businesses such as electric vehicle charging infrastructure services and smartphone apps. Since 2015, he has been in charge of investment at Kyoto University Innovation Capital Co., Ltd. He joined Shinobi Therapeutics in 2020, where he now leads the business and finance aspects of the company. He holds a B.A. in Law from Kyoto University and an MBA from Carnegie Mellon University.
Company overview
- Name
- :Shinobi Therapeutics Co. Ltd.
- President and Representative Director
- :Takafumi Yano
- Representative Director
- :Ryosuke Gonotsubo
- Established
- :August 24, 2015
- Address
- :46-29 Yoshida-Shimoadachicho, Sakyo-ku, Kyoto
- Business activities
- :Research and development of iPS cell-derived immune cells therapy with hypoimmune technology
- Website
- :https://www.shinobitx.com/






